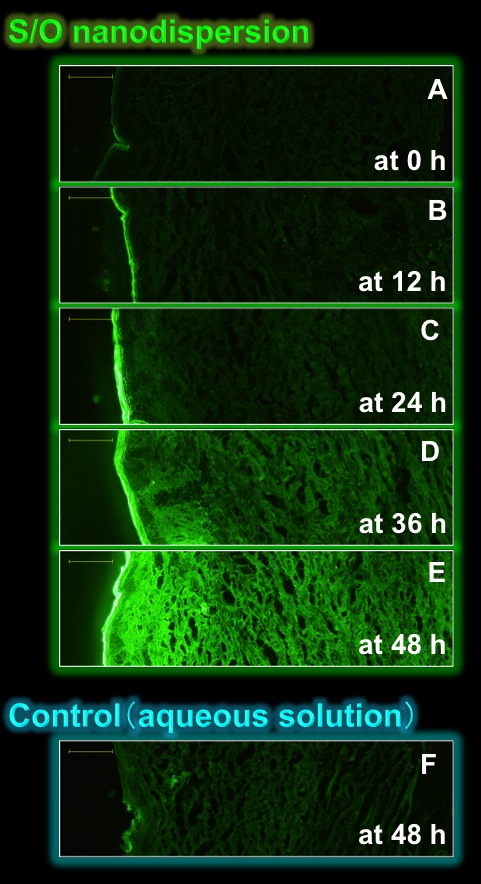
 Fig.3 Fluorescence microscopy
treated with the samples
containing FITC-labeled insulin [9].
|
2.2. Transcutaneous drug delivery
A transcutaneous drug delivery, drug delivery through the skin, has many advantages over injection or oral administration because it can avoid first-pass hepatic metabolism and provides the patients with an easier and more convenient route for drug administration. Despite its great potential, the delivery of hydrophilic macromolecules such as peptides and proteins through the skin remains a challenging issue in the development of drug delivery systems. This is mainly due to the intrinsic barrier function of the skin, provided by the highly organized structure of the stratum corneum. Therefore, a number of chemical penetration enhancers have been employed to increase the permeability of the drugs into the skin. However, it has been difficult to achieve therapeutic levels of relatively large drugs (over 500 Da) through intact skin to the systemic circulation by the chemical penetration enhancer alone.
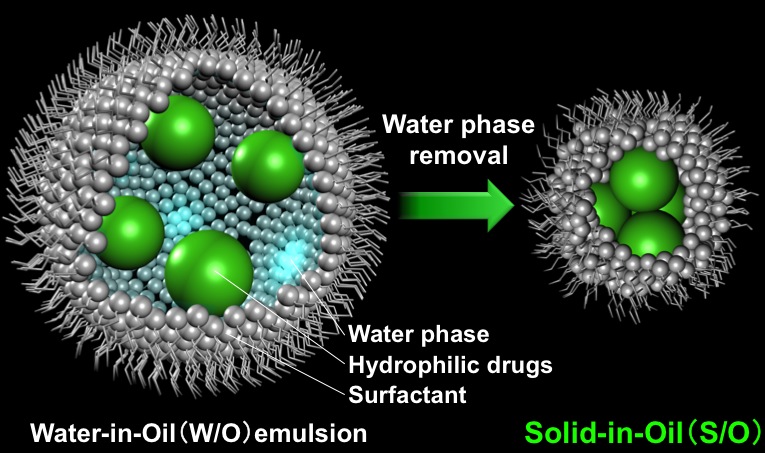
To overcome these situations, Goto laboratory proposed a novel transcutaneous drug delivery system by a S/O nanodispersion technique. The idea is that the dispersion of the proteins modified with the surfanctant in the oil phase makes the proteins permeable into the skin without any physical enhancers or pre-treatments if a suitable oil with the properties of a chemical penetration enhancer is selected. This concept is novel because to date, almost all studies on transcutaneous delivery of hydrophilic drugs have been based on aqueous vehicles.
Fig.3 shows fluorescent images of the micropig skin sections treated with the S/O nanodispersion or the control (aqueous solution) containing FITC-labeled insulin. The green fluorescence derived from the FITC-labeled insulin was gradually increased with time when the S/O nanodispersion was applied (Fig.3A-E). By contrast, little fluorescence was observed with the aqueous counterpart (Fig.3F). Further, we demonstrated that proteins up to 40 kDa in molecular weight can be penetrated into the skin by the newly developed S/O nanodispersion technique. Although there still remain the main limitations such as slow permeation through the skin, the S/O nanodispersion technique has a high potential for the creation of a novel transcutaneous protein delivery system.[9]
2.3. Transcutaneous immunization
Coming soon...
|
|
3. Solid-in-Oil-in-Water (S/O/W) emulsion
We have developed the oral protein delivery by a Solid-in-Oil-in-Water (S/O/W) emulsion. This section is under construction!!
4. Gene delivery
We have developed the a novel non-viral gene vector by our unique emulsion. This section is under construction!!
5. Collaborative work
If you are interested in our research, please contact with Prof. Goto or SO pharmaceutical corp..

6. References
- E. Toorisaka, H. Ono, K. Arimori, N. Kamiya, M. Goto, "Hypoglycemic effect of surfactant-coated insulin solubilized in a novel solid-in-oil-in-water (S/O/W) emulsion" Int. J. Pharm., 252, 271-274 (2003).@
- ΚγhκC_κDqC_JTδCγ‘λGAW/O/W^½G}VΙό΅½RKά_CmeJΜRk@\A»wHw_ΆWCζ29ͺCζ2Cpp.294-297 (2003)
- E. Toorisaka, M. Hashida, N. Kamiya, H. Ono, Y. Kokazu, M. Goto, "An enteric-coated dry emulsion formulation for oral insulin delivery" J. Control. Release, 107, 91-96 (2005).
- H. Piao, N. Kamiya, J. Watanabe, H. Yokoyama, A. Hirata, T. Fujii, I. Shimizu, S. Ito, M. Goto, "Oral delivery of diclofenac sodium using a novel solid-in-oil suspension" Int. J. Pharm., 313, 159-162(2006).
- H. Piao, N. Kamiya, A. Hirata, H. Yokoyama, T. Fujii, I. Shimizu, S. Ito, M. Goto, "Reduction of gastric ulcerogenicity during multiple administration of diclofenac sodium by a novel solid-in-oil suspension" Pharm. Dev. Technol., 12, 321-325 (2007).
- H. Yoshiura, M. Hashida, N. Kamiya, M. Goto, "Factors affecting protein release behavior from surfactant.protein complexes under physiological conditions" Int. J. Pharm., 338 174-179 (2007).
- H. Yoshiura, Y. Tahara, M. Hashida, N. Kamiya, A. Hirata, T. Fujii, M. Goto, "Design and in vivo evaluation of solid-in-oil suspension for oral delivery of human growth hormone" Biochem. Eng. J., 41 106-110 (2008).
- H. Piao, N. Kamiya, A. Hirata, T. Fujii, I. Shimizu, S. Ito, M. Goto, "A novel solid-in-oil nanosuspension for transdermal delivery of diclofenac sodium" Pharm. Res., 25 896-901 (2008).
- Y. Tahara, S. Honda, N. Kamiya, H. Piao, A. Hirata, E. Hayakawa, T. Fujii, M. Goto, "A solid-in-oil nanodispersion for transcutaneous protein delivery" J. Control. Release, 131, 14-18 (2008).@[ Fig.1 , Fig.2 , Fig.3 are discribed]
- c΄`NA_JTδAγ‘λGAu^pNΏΜoηfo[Μΐ»vAoCITCGXΖC_Xg[igsbNXjA67(2)ͺAp.68-70 (2009)
- γ‘λGAμh‘A½c²FARΊA
μPAΝ΄΄ΝAφT[Auς¨ΜimR[`O(S/O)Zpπp΅½»ΟiASPION CEvAiMembrane)A34(3)ͺAp.159-161@(2009)
- εF€qAp^FAc΄`NA_JTδAγ‘λGAuSolid-in-Oil»Zpπp΅½AXRr_U±ΜΜoηfo[VXevAiMembrane)A34(4)ͺAp.227-232@(2009)
- c΄`NA_JTδAγ‘λGAuS/O»ZpΜ£ΝΖV΅’oης¨BVXeΐ»ΜΒ\«vAPHARM TECH JAPANA25(7)ͺAp.1409-1414@(2009)
Top page
|